Completing the Loop
Friday, December 4, 2009
Summary
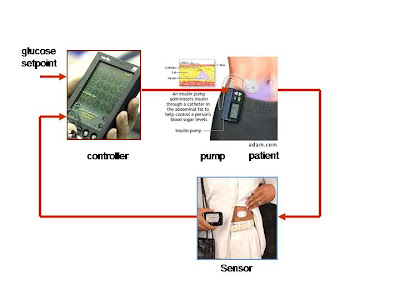
So what are researchers doing to improve this imperfect and tedious method for regulating blood glucose? The answer seems to lie in a device called an artificial pancreas, which hopefully will ultimately function as an effective regulator of blood glucose levels. Here is how the technology works: an iPod-sized pancreas is worn outside the body and consists of two main parts, a continuous glucose monitor and an insulin pump. A computer chip embedded in the insulin pump takes in information about the patient’s blood sugar levels from a glucose monitor which is attached to the skin. Algorithms and sensors are used to predict and determine what insulin levels are in the body and the necessary dosage to be released. When each component is combined to “close the loop,” a more effective system for controlling glyemic levels is possible. This means that, in theory, diabetic patients will be able to eat whatever they want, whenever they want, without having to think twice about what their blood glucose levels are; the artificial pancreas will do all of the thinking and monitoring for them.
However, the device is currently far from perfect, and experts estimate that it will not be available full swing on the market for another 5-10 years. Why? Here are some of the problems: An automated pump shut-off needs to be added to the system, as well as a device for “hybrid” glucose control. “Hybrid” glucose control refers to a new attempt at regulating infusion rates, after researchers noted that the controlling algorithm was leaving significant postprandial excursions with the system because of delays in insulin absorption. Further refinements are still necessary to accelerate insulin action and decay rates to minimize postprandial glycemic excursions and reduce late postprandial hypoglycemia. Furthermore, researchers are debating whether the inclusion of glucogen in the system is necessary or even feasible. Some are convinced that a counter-regulatory hormone is needed because huge drops in blood sugar can happen due to changes in insulin sensitivity in a very short amount of time.
Thus while many patients in clinical trials have found immense success with the device, there are many obstacles which first must be overcome. If anything were to go wrong with the device when people were fully dependent on it, the results could be disastrous.
As a group we believe that this device may be very effective for the majority of the Diabetes population. It would provide a greater ability to maintain blood glucose levels in the range doctors advise, and would be of greater convenience to those using it than the traditional methods used by patients. For some, price would seem to be an issue, but at this point it is not clear what the cost for the closed-loop system would be and if insurance would cover it. Many studies and trials on the system have shown that it’s a major step up from current technology in effectiveness for preventing hypo/hyperglycemia. But there are still many issues as stated in the previous articles and commentaries. The algorithm being used obviously needs to be improved and there are questions on whether there should be any interface between the person using it and the system itself. These types of issues are constantly being worked on and it looks as if they will be solved in the very near future.
One large question that we feel is important is: How would this device improve the quality of life for patients? We’ve seen some trial responses to this. In the case of Julie Anne Ressler, she explained how life was exceptionally better with the artificial pancreas and that she was lucky to have gotten the chance to try it for at least a few days. In order to determine success in these terms, the human costs and effects need to be weighed just as much as the actual medicine and science; and in most cases it has been worthwhile for the patients to use the system. So, if there are so many positive results and responses to the Artificial Pancreas, why has it not yet been approved by the FDA for commercial use? Well the answer simply comes from what was mentioned earlier. An imperfect computing algorithm tends to be the most troublesome along with issues with interface and sensitivity of the sensors being used.
Overall, it cannot be argued for certain that the days of greater lives for Diabetes patients may be coming very soon. Who knows how much more this technology will improve in the next few years and beyond? Scientists and proponents of our biomedical system are always optimistic when discussing new technologies, but history has shown us that perfecting new technologies is often a long and tedious process. But one thing is certain, and that is the undeniable truth of how effective this system has been in individual patient trials, regardless of its imperfections. No matter what critics may say (and it does not seem there are many critics, thus further proving the mechanism’s potential), trials have given researchers assurance that the technology will work. Now we must sit back and wait until this is presented to the public and watch as it helps change lives.
After reading extensive literature on the artificial pancreas, we still wanted a firsthand source from an expert on the subject. While we believed the articles we read were reliable, we were a bit concerned that they may have been slightly too optimistic. Thus we interviewed Michael Dempsey, MD, a practicing endocrinologist in the DC area. Here is what we found:
1. Overall, how would you assess the artificial pancreas? ( On a scale from 1-10, 10 being strongly efficient and 0 being completely inefficient).
I would rate it at a 5. There are several problems. The first is the lack of reliability and reproducibility of blood glucose (BG) results from continuous glucose monitoring. The entire system depends on accurate BG measurements. If BG measurements aren't correct, inappropriate insulin doses may be given. Size is also a concern. To be a reasonable choice for patients, the units have to be small. Infection is a concern if the pumps are implanted, as is the need for re-operation. Fibrosis is also a concern if implanted. If this becomes severe, removal and re-insertion will be required. Finally, there are prohibitive legal concerns. If an artificial pancreas malfunctioned and caused severe hypoglycemia, what would happen, for example, if the patient was driving and killed someone? This would be a huge setback to research on the technology.
2. Once the FDA fully approves the device, will you urge your patients to use it? Why or why not?
I will be cautious due to concerns about safety and costs. Why would an insurance company agree to pay for this unless the patient can prove that this device will result in better BG control than current, less expensive alternatives such as multiple daily injections of insulin? I would also need proof that glucose control is better with these units.
4. In all of the articles we have come across, none mention the expected price of the new device? Any guesses? How will this be covered?
A conventional pump costs $5,000-$6,000. I would estimate that a closed loop system would cost $10,000-$15,000. If it was implanted, you would also need to account for surgical costs which could range from $3,000-$5,000. I think it is unlikely that insurers will agree to cover this unless there is proof that a patient has failed all conventional insulin regimens. There might be coverage for patients with documented recurrent severe hypoglycemia (low BG with loc or seizures) while using conventional regimens or those who require immediate intensive therapy such as pregnant type-1 (T1), or transplant patients.
5. How many times a day do people with Type 1 Diabetes usually check their glucose levels?
The average is 3 times per day (pre-meals). Some will check 6 or more times per day. Women with T1 who are pregnant test before each meal, 1 hour after each meal, and occasionally in the middle of the night.
6. Do you think the artificial pancreas will eliminate the constant reminder of glucose levels completely?
No. People will still need to calibrate the pancreas with FS BG testing. It would decrease testing, however, to perhaps 2 times per day.
7. What types of improvements would you make for the up and coming closed loop system? Improved reliability of the continuous BG monitoring component of the artificial pancreas.
8. Would you feel comfortable giving the artificial pancreas to children? It is said to be good for removing the responsibility of monitoring numbers and times, but how do you feel regarding this issue?
It would depend on the child and the family. If it is a bright child with motivated, involved parents, yes. Otherwise, I would be cautious.
9. With regenerative medicine (honing in on the Edmonton protocol) and the development of the artificial pancreas, do you think the treatment of Type 1 Diabetes is embarking on a huge leap forward? Please explain.
I don't see this technology as being practical for most T1's due to cost concerns. Re-transplantation (the Edmonton protocol), is always limited by availability of donor pancreases. There may also be problems with the need for immunosuppression (this will increase risk of infections), even with this protocol. I am more interested in monoclonal antibody protocols. This involves production of antibodies directed against the T-cells that destroy the beta cell. Tolerx and Macrogenics are companies involved in these trials. By "putting the T-cells to sleep", you can preserve beta cell function. Infection risk is not increased. If you preserve beta cell function, the diabetes may be easier to manage. There is also some data that patients with preserved beta cell function have fewer small vessel complications such as diabetic eye disease (retinopathy).
10. Is it true that one of the major functions of the artificial pancreas is that it will decrease the frequency of hypoglycemic reactions throughout the night?
Yes. It would eliminate BG variability, both highs and lows.
11. Can you think of any negative side effects of using the artificial pancreas? If blood glucose is measured incorrectly by the continuous monitor, severe, prolonged hypoglycemia (due to too much insulin) or severe hyperglycemia (due to too little insulin) could result. If the unit stopped delivering insulin, and the warning systems didn't alert the patient, the BG levels could rise rapidly to very dangerous levels (diabetic ketoacidosis or DKA). There are also the risks of infection and the need for re-operation if the unit malfunctions.
12. Is there a specific population of Type 1 Diabetes patients who you would strongly advise to use the artificial pancreas? Yes. Patients unable to control their diabetes mellitus (DM) with conventional intensive regimens (a very small number), and those patients interested in the technology who were bright and have plenty of money! If the devices were external, I would also consider patients who needed very tight BG control such as pregnant T1’s.
13. As Brown students, we love to think out of the box, so, we are wondering, what is next, Doctor Dempsey? Delve into your wildest dreams and please inform us of what you think scientists will come up with next in order to continue the defeat of Type 1 Diabetes. World-wide screening of non-diabetics for T1 diabetic markers such as GAD-65 and islet cell antibodies. If positive, treat the people at risk with monoclonal antibodies to prevent the development of T1 DM. Rapid advances in continuous BG monitoring to allow accurate testing of BG without forcing patients to constantly check their BG. The ability to grow human beta cells, along with the ability to prevent their destruction once implanted into people with T1 DM.
This interview gave us a very reasoned, practical perspective into the new technology of the artificial pancreas. Although it did confirm our belief (to a certain extent) that some of the articles we read may have been a bit on the optimistic side, again this is only one professional opinion and others may vary. We are still optimistic about this technology.
Next we wanted to interview someone on the opposite end of the spectrum, someone who might be a potential candidate for having an artificial pancreas. Thus we interviewed Ziad Kharbush, a 19-year-old sophomore at Brown University who is a wrestler on the varsity wrestling team and who plans to concentrate in biology. Here is what we found out:
1. When were you diagnosed with type 1 Diabetes? I was diagnosed on March 21, 2005, when I was turning 16.
2. How would you describe the adjustment to type 1 Diabetes? I would definitely say that it’s tedious and aggravating
3. What has been the hardest thing to get used to? The worst part is getting used to the way that it messes with my energy. It’s pretty annoying.
4. What do you think about the artificial pancreas? I think it looks really cool. I remember taking my first shot ever and telling myself, I don't want to have to do this for the rest of my life.
5. Do you plan to use the artificial pancreas? Yes, I’ll use it once it's more efficient and completely safe. I also can’t be playing a full-contact sport (wrestling) if I’m using it.
6. Has diabetes changed your life? If so, how? Yes, I am more careful now. Plus, again, my energy fluctuates so much that everything is a bit harder and more tedious. This is especially difficult since I wrestle, and obviously, you must have a lot of energy when competing in matches, practicing, etc.
7. Since your life has changed, do you think your life will get better with the pancreas? Absolutely yes.
8. What feature are you most excited about with the artificial pancreas? I am most excited about not having to test constantly. It doesn’t seem like a big deal to most people who don’t have diabetes, but it’s always on your mind, and when you have to do it everyday of your life it can get a bit annoying.
Ziad’s optimistic attitude reflects that of many diabetic patients. Soon, hopefully, diabetic patients everywhere will see these hopes fulfilled.
Monday, November 23, 2009
Costly or Not?
In most articles I've come across, there is no specific price which is given as an estimate for the device, but most seem hopeful for the price to be right around that of the CGM. Also, the JDRF is pushing towards attempting to push for insurance coverage of the device once it is approved for use by the public.
Also, speaking in terms of overall cost, this product would greatly reduce the amound patients would spend treating their Diabetes, it would be very cost-effective in the long term.
Here are a couple links which lightly discuss this topic:
http://artificialpancreasproject.com/faq/#approximately-how-much - this link also answers many questions which one may have about the device, it's development, etc..
http://web2100.jou.ufl.edu/index.php?id=1237
Sunday, November 22, 2009
Artificial Pancreas Just Years Away, Experts Agree
Maggie Fox July 26, 2008
This article focuses on the timeframe for when an artificial pancreas may come full-swing to market. The general consensus among researchers working on the project is that a fully-functioning artificial pancreas will be on the market within five years. Currently, many clinical trials have shown that the artificial pancreas is near perfect, with the new pancreas regulating glucose levels much more thoroughly than that in the diabetic patient’s body. As people with diabetes cannot constantly check their glucose levels, generally they are limited to checking at most twenty times a day. While the artificial pancreas is not perfect yet, it monitors glucose levels continuously and thus still may function better than the actual, bodily pancreas, particularly for young children who cannot check insulin levels themselves and thus require constant parent supervision. But before the new technology can be marketed, several of its quirks must first be fixed. Researchers are confident that these will be fixed in several years, but many diabetic patients are extremely anxious for this new technology to reach the market, having found immense success in clinical trials.
"Artificial Pancreas" For Some Diabetics
In Summer 2008, CBS News did a story on Julie Anne Ressler. She is a mom, a doctor, and a Type 1 diabetic. For the majority of her life, she has had to prick her finger more than 10 times a day in order to monitor her blood glucose levels and to determine how much insulin she should take. She says that she is constantly reminded throughout the day that she has diabetes because she has to check her numbers so much. Recently, in Los Angeles, she was put on a test trial with the artificial pancreas. The doctor who used her for the test trial said she loved it because she wasn't worried about her diabetes all day; the artificial pancreas took care of everything. Without any direction from herself, the machine was able to successfully detect blood glucose levels and then give the appropriate amount of insulin. Again, as mentioned in previous posts, everyone is anticipating the approval of the artificial pancreas by the FDA.
In order for biotechnology to be practiced effectively, the human costs and affects need to be weighed just as much as the actual medicine and science. This CBS show elucidates this point; it hones in on the human perspective of the artificial pancreas. Julie Anne Ressler explained how life was exceptionally better with the artificial pancreas and that she was lucky to have gotten the chance to try it for at least a few days.
With this in mind, I was upset that with positive patient feedback, the FDA still had not given the artificial pancreas approval. I decided to look at the FDA website for more information regarding the much anticipated artificial pancreas approval. In order to expedite the approval, the FDA has created the Interagency Artificial Pancreas Working Group (IAPWG). This group works with private organizations, patient groups, academic researchers, product developers, industry and other government groups in order to figure out ways to speed up research and development. Most of the problems that are interfering with approval involve technological difficulties ( ie imperfect algorithims and mismatches between blood and interstitial glucose levels)
Here is the CBS showing about Julie Anne Ressler and the artificial pancreas.
http://www.cbsnews.com/sections/i_video/main500251.shtml?id=4327391n
http://www.cbsnews.com/stories/2008/08/06/earlyshow/main4324074.shtml
http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ArticlesandPresentations/ucm077537.htm
Medtronic begins international sales of semi-closed loop Paradigm Veo diabetes device
http://www.medcitynews.com/index.php/2009/09/medtronic-begins-international-sales-semi-closed-loop-paradigm-veo-diabetes-device/
Saturday, November 21, 2009
Researchers Outline Steps to Artificial Pancreas
In an article from Critical Endocrinology News, information is presented on the steps towards closing the loop in the Artificial Pancreas Project. The JDRF has been working to solve many issues which have been problematic to completing this project. These include adding an automated pump shut-off, having "hybrid" glucose cont
 rol, the possible inclusion of glucogen, silico modeling and incorporating an in-hospital closed loop system.
rol, the possible inclusion of glucogen, silico modeling and incorporating an in-hospital closed loop system.The automated pump shut-off will control the information sent from the sensor to shut off insulin delivery; this whole process will help to prevent hypo/hyper glycemic levels in patients.
"Hybrid" glucose control refers to a new attempt at regulating infusion rates. Dr. Stuart Weinzimer of the department of pediatrics at Yale University and his associates found that the controlling algorithm was leaving significant postprandial excursions with the system because of delays in insulin absorption. Weinzimer and his associates proposed introducing small manual priming doses prior to meals during close looped control. The results were not quite what the researchers expected, but they are optimistic about the feasibility of the closed loop control with the interaction of manual doses. “Further refinements are necessary to accelerate insulin action and decay rates to minimize postprandial glycemic excursions and reduce late postprandial hypoglycemia,” said Weinzimer.
Inclusion of glucogen is a debatable area, but according to Edward Damiano, Ph.D, of the division of Biomedical Engineering at Boston University, the introduction of glucogen in a truly closed loop system is neccessary. “I’m pretty convinced that you need a counterregulatory hormone. In real life, tremendous precipitous drops in blood sugar can happen due to changes in insulin sensitivity in a very short space of time. ... There’s no way a machine can prevent something like that, that quickly. Glucagon works extremely fast,” he said.
Another topic, in silico modeling refers to computer simulation of patient and device variables. Typically, there is a need to study animals before humans, but this is costly and time consuming. These computer simulations can rapidly assess the feasibility of various algorithms for human trials and therfore save years of time which would have been spent on testing.
Finally, in-hospital closed loop systems could be very beneficial for hospitalized patients. It would help to take strain off of nursing staffs who cannot manage to closely monitor patients continuously. And since most initial clinical trials have taken place in hospital settings, it would not be an uncommon practice.
All in all, these topics are among the final obstacles and decisions which must be solved and determined for the completion of the closed loop system. Just a few steps now stand between us and a functional Artificial Pancreas.
http://www.jdrf.org/files/General_Files/APP/2008/Clinicalendocrinologynews_AP.pdf
Sunday, November 15, 2009
“Latest Advance in the Treatment of Diabetes: An Artificial Pancreas”
After reading the November 2008 article (“Researchers Developing Artificial Pancreas to Treat Diabetes”), I wanted to find a more recent article which might shed some insight on new developments with the artificial pancreas. This article, published in July of 2009, focuses on the life of a 14-year-old girl, Sarah, who has briefly tried using an artificial pancreas and has experienced amazing results. According to the article, Sarah must test her blood sugar ten or more times a day, as well as count her carbohydrate intake and adjust the amount of insulin she gives herself based on this information. But for a few days Sarah didn’t have to worry about such tedious measures. Instead she was able to test out an experimental artificial pancreas, one of approximately 75 diabetes patients across the U.S. taking part in the clinical trials. Every minute, the artificial pancreas delivers the necessary amount of insulin to control sugar levels. As 7.8 percent of the U.S. population has diabetes, a device which could procure these amazing results would dramatically improve the lives of millions. Currently, some diabetic patients already have continuous sensors which monitor sugar levels in the body, as well as pumps that dispense insulin. The goal of the new artificial pancreas is to link these two components. With the new technology, a sensor beneath the skin sends a signal to the transmitter, which then goes to a control box. The control box in turn tells the pump how much insulin to release. The patient feels nothing while this occurs, and can eat whatever she wants while the artificial pancreas makes all of the adjustments. But the technology is far from perfect, as researchers are now trying to make the device smaller and more precise.
http://abcnews.go.com/Health/MedicineCuttingEdge/story?id=8043506&page=1
“Researchers Developing Artificial Pancreas to Treat Diabetes”
This article from Scientific American provides a basic overview of the current artificial pancreas technology, although it must be noted that the article was written several months ago and therefore the technology may have improved slightly since then. Patients with diabetes must constantly attempt to maintain the right blood sugar level, a difficult and time-consuming job. These patients make little or no insulin, a hormone which is normally produced in the pancreas and which breaks down food into energy. The problem with insulin injections is that they prevent too much sugar from accumulating in the blood, potentially leading to a diabetes-induced coma. Therefore scientists in England and the United States are working on an artificial, iPod-sized pancreas to be worn outside the body. The artificial pancreas consists of two main parts: a continuous glucose monitor and an insulin pump. A computer chip embedded in the insulin pump takes in information about the patient’s blood sugar levels from a glucose monitor which then attaches to the skin. The pump would be worn on a belt or in a pocket and would use the information to inject the necessary amount of insulin through a needle in the belly. This would allow the patient to have the right amount of insulin in her blood without having to manually change the dosage. The projected cost of the technology has not yet been determined, and Bruce Buckingham of Stanford University, where some of the research is occurring, guesses that the device is still five to ten years away from the market. Devices made by the companies Medtronic Diabetes, Abbott Laboratories, and Johnson & Johnson are testing the software. Although the technology is undoubtedly promising, in order to truly be effective it must be provided at a relatively low cost; otherwise, the millions of people who would benefit may not have access. And if universal healthcare is implemented, will the artificial pancreas be provided for? How will the accessibility differ between different age groups? These are the types of questions which must be addressed in order to determine the feasibility of this new technology.
http://www.scientificamerican.com/blog/post.cfm?id=researchers-developing-artificial-p-2008-11-03
An Artificial Pancreas
http://www.technologyreview.com/biomedicine/21196/page2/
Artificial Pancreas Could Revolutionize Treatment Of Type 1 Diabetes
The algorithim, also known as the "smart program" proved to be exceedingly complicated. Researchers in the US and Italy were trying to create a simple program which would be able to pick up the host blood glucose level, and then somehow go into the database and pick out the exact dosage of insulin which would suit the numbers. Basically, these medical engineers needed to devise a system which could compute on a human- like level.
After countless months in development, the FDA stepped in. While developing the silico computer simulation experiments, the FDA granted the researchers approval to test the novel artifical pancreas in humans before the animal trials. This cut the overall development process from several years to six months.
The fact that the FDA eliminated in vivo trials says alot about the artifical pancreas in a biotechnological light. When new technologies and drugs are expedited like this, it signifies the importance and necesity of either. Cancer drugs are often expedited during development because they are extremely needed for cancer patients to maintain health, just like this artificial pancreas; it is absolutely mandatory in regulating Type 1 Diabetes.
With the devotion of research teams around the world, and the involvement of the FDA, I think the artificial pancreas will soon prove to be the best way to treat Type 1 Diabetes. I think in a few years, the artificial pancreas will be less expensive because so many people will need/ want them.
http://www.medicalnewstoday.com/articles/126827.php
Fully Closed
The major variable of the experiment was testing blood glucose levels at various points during the day. They also measured A1C levels and the frequency of hypoglycemic events. The results proved that the variation between the fully closed and hybrid loop were minimal (1 to 2 points). The fully closed system did nevertheless prove to be slightly more efficient. This was due to the fact that the insulin pump could administer pre-meal bolus doses which broke down the initial insulin resistance and smoothed out the blood sugar fluctuation.
Glucose levels with FCL control almost never exceeded 300 mg/dl. 85% of all sensor glucose levels were between 70 and 180 mg/dl in the FCL group during the 24-hour study period. During standard open-loop pump therapy, during which time only 58% of sensor glucose values were between 70 and 180 mg/dl. These subjects were already in excellent control, with mean A1C level of only 7.1%.
Hypoglycemia is a main concern as the best blood sugar control comes with the price of potential hypoglycemia. Hypoglycemia occurred only at nighttime in the experiment. Two patients of the Hybrid control group suffered hypoglycemic events in which blood glucose levels reached 57 (mg/dl). The fully closed loop control group patient reached 51 (mg/dl). These episodes were easily fixed with 15g of carbohydrates.
http://care.diabetesjournals.org/content/31/5/934.full#sec-13
"Automated 'Artificial Pancreas' Controls Blood Glucose Levels In Diabetes Patients For First Time
http://www.medicalnewstoday.com/articles/153002.php
Friday, November 13, 2009
"Closing the loop: Artificial pancreas may be just a few years away"
http://www.endocrinetoday.com/view.aspx?rid=43543
