Currently, an astounding 23.6 million people in the United States have diabetes. This equates to approximately 7.8% of the entire population, a staggering statistic. Diabetes was the seventh leading cause of death listed on US death certificates in 2006, and approximately $174 billion was the total cost of diagnosed cases of diabetes in 2007. With these numbers in mind, it is clear that diabetes is no small issue. Those who are unlucky enough to have the disease must constantly monitor their blood glucose levels every day, 365 days a year. This requires that they prick their fingers at constant intervals, an extremely annoying and tedious process when it must be done repeatedly. Furthermore, the process is not entirely effective or even safe, as blood glucose levels fluctuate with insulin injections, preventing too much sugar from accumulating in the blood and potentially leading to a diabetes-induced coma and distress in other parts of the body.
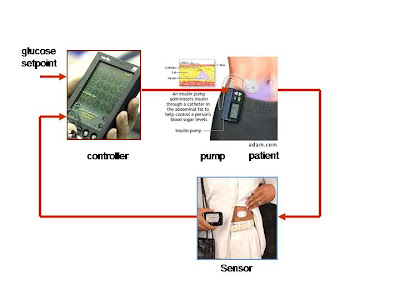
So what are researchers doing to improve this imperfect and tedious method for regulating blood glucose? The answer seems to lie in a device called an artificial pancreas, which hopefully will ultimately function as an effective regulator of blood glucose levels. Here is how the technology works: an iPod-sized pancreas is worn outside the body and consists of two main parts, a continuous glucose monitor and an insulin pump. A computer chip embedded in the insulin pump takes in information about the patient’s blood sugar levels from a glucose monitor which is attached to the skin. Algorithms and sensors are used to predict and determine what insulin levels are in the body and the necessary dosage to be released. When each component is combined to “close the loop,” a more effective system for controlling glyemic levels is possible. This means that, in theory, diabetic patients will be able to eat whatever they want, whenever they want, without having to think twice about what their blood glucose levels are; the artificial pancreas will do all of the thinking and monitoring for them.
However, the device is currently far from perfect, and experts estimate that it will not be available full swing on the market for another 5-10 years. Why? Here are some of the problems: An automated pump shut-off needs to be added to the system, as well as a device for “hybrid” glucose control. “Hybrid” glucose control refers to a new attempt at regulating infusion rates, after researchers noted that the controlling algorithm was leaving significant postprandial excursions with the system because of delays in insulin absorption. Further refinements are still necessary to accelerate insulin action and decay rates to minimize postprandial glycemic excursions and reduce late postprandial hypoglycemia. Furthermore, researchers are debating whether the inclusion of glucogen in the system is necessary or even feasible. Some are convinced that a counter-regulatory hormone is needed because huge drops in blood sugar can happen due to changes in insulin sensitivity in a very short amount of time.
Thus while many patients in clinical trials have found immense success with the device, there are many obstacles which first must be overcome. If anything were to go wrong with the device when people were fully dependent on it, the results could be disastrous.
As a group we believe that this device may be very effective for the majority of the Diabetes population. It would provide a greater ability to maintain blood glucose levels in the range doctors advise, and would be of greater convenience to those using it than the traditional methods used by patients. For some, price would seem to be an issue, but at this point it is not clear what the cost for the closed-loop system would be and if insurance would cover it. Many studies and trials on the system have shown that it’s a major step up from current technology in effectiveness for preventing hypo/hyperglycemia. But there are still many issues as stated in the previous articles and commentaries. The algorithm being used obviously needs to be improved and there are questions on whether there should be any interface between the person using it and the system itself. These types of issues are constantly being worked on and it looks as if they will be solved in the very near future.
One large question that we feel is important is: How would this device improve the quality of life for patients? We’ve seen some trial responses to this. In the case of Julie Anne Ressler, she explained how life was exceptionally better with the artificial pancreas and that she was lucky to have gotten the chance to try it for at least a few days. In order to determine success in these terms, the human costs and effects need to be weighed just as much as the actual medicine and science; and in most cases it has been worthwhile for the patients to use the system. So, if there are so many positive results and responses to the Artificial Pancreas, why has it not yet been approved by the FDA for commercial use? Well the answer simply comes from what was mentioned earlier. An imperfect computing algorithm tends to be the most troublesome along with issues with interface and sensitivity of the sensors being used.
Overall, it cannot be argued for certain that the days of greater lives for Diabetes patients may be coming very soon. Who knows how much more this technology will improve in the next few years and beyond? Scientists and proponents of our biomedical system are always optimistic when discussing new technologies, but history has shown us that perfecting new technologies is often a long and tedious process. But one thing is certain, and that is the undeniable truth of how effective this system has been in individual patient trials, regardless of its imperfections. No matter what critics may say (and it does not seem there are many critics, thus further proving the mechanism’s potential), trials have given researchers assurance that the technology will work. Now we must sit back and wait until this is presented to the public and watch as it helps change lives.
After reading extensive literature on the artificial pancreas, we still wanted a firsthand source from an expert on the subject. While we believed the articles we read were reliable, we were a bit concerned that they may have been slightly too optimistic. Thus we interviewed Michael Dempsey, MD, a practicing endocrinologist in the DC area. Here is what we found:
1. Overall, how would you assess the artificial pancreas? ( On a scale from 1-10, 10 being strongly efficient and 0 being completely inefficient).
I would rate it at a 5. There are several problems. The first is the lack of reliability and reproducibility of blood glucose (BG) results from continuous glucose monitoring. The entire system depends on accurate BG measurements. If BG measurements aren't correct, inappropriate insulin doses may be given. Size is also a concern. To be a reasonable choice for patients, the units have to be small. Infection is a concern if the pumps are implanted, as is the need for re-operation. Fibrosis is also a concern if implanted. If this becomes severe, removal and re-insertion will be required. Finally, there are prohibitive legal concerns. If an artificial pancreas malfunctioned and caused severe hypoglycemia, what would happen, for example, if the patient was driving and killed someone? This would be a huge setback to research on the technology.
2. Once the FDA fully approves the device, will you urge your patients to use it? Why or why not?
I will be cautious due to concerns about safety and costs. Why would an insurance company agree to pay for this unless the patient can prove that this device will result in better BG control than current, less expensive alternatives such as multiple daily injections of insulin? I would also need proof that glucose control is better with these units.
4. In all of the articles we have come across, none mention the expected price of the new device? Any guesses? How will this be covered?
A conventional pump costs $5,000-$6,000. I would estimate that a closed loop system would cost $10,000-$15,000. If it was implanted, you would also need to account for surgical costs which could range from $3,000-$5,000. I think it is unlikely that insurers will agree to cover this unless there is proof that a patient has failed all conventional insulin regimens. There might be coverage for patients with documented recurrent severe hypoglycemia (low BG with loc or seizures) while using conventional regimens or those who require immediate intensive therapy such as pregnant type-1 (T1), or transplant patients.
5. How many times a day do people with Type 1 Diabetes usually check their glucose levels?
The average is 3 times per day (pre-meals). Some will check 6 or more times per day. Women with T1 who are pregnant test before each meal, 1 hour after each meal, and occasionally in the middle of the night.
6. Do you think the artificial pancreas will eliminate the constant reminder of glucose levels completely?
No. People will still need to calibrate the pancreas with FS BG testing. It would decrease testing, however, to perhaps 2 times per day.
7. What types of improvements would you make for the up and coming closed loop system? Improved reliability of the continuous BG monitoring component of the artificial pancreas.
8. Would you feel comfortable giving the artificial pancreas to children? It is said to be good for removing the responsibility of monitoring numbers and times, but how do you feel regarding this issue?
It would depend on the child and the family. If it is a bright child with motivated, involved parents, yes. Otherwise, I would be cautious.
9. With regenerative medicine (honing in on the Edmonton protocol) and the development of the artificial pancreas, do you think the treatment of Type 1 Diabetes is embarking on a huge leap forward? Please explain.
I don't see this technology as being practical for most T1's due to cost concerns. Re-transplantation (the Edmonton protocol), is always limited by availability of donor pancreases. There may also be problems with the need for immunosuppression (this will increase risk of infections), even with this protocol. I am more interested in monoclonal antibody protocols. This involves production of antibodies directed against the T-cells that destroy the beta cell. Tolerx and Macrogenics are companies involved in these trials. By "putting the T-cells to sleep", you can preserve beta cell function. Infection risk is not increased. If you preserve beta cell function, the diabetes may be easier to manage. There is also some data that patients with preserved beta cell function have fewer small vessel complications such as diabetic eye disease (retinopathy).
10. Is it true that one of the major functions of the artificial pancreas is that it will decrease the frequency of hypoglycemic reactions throughout the night?
Yes. It would eliminate BG variability, both highs and lows.
11. Can you think of any negative side effects of using the artificial pancreas? If blood glucose is measured incorrectly by the continuous monitor, severe, prolonged hypoglycemia (due to too much insulin) or severe hyperglycemia (due to too little insulin) could result. If the unit stopped delivering insulin, and the warning systems didn't alert the patient, the BG levels could rise rapidly to very dangerous levels (diabetic ketoacidosis or DKA). There are also the risks of infection and the need for re-operation if the unit malfunctions.
12. Is there a specific population of Type 1 Diabetes patients who you would strongly advise to use the artificial pancreas? Yes. Patients unable to control their diabetes mellitus (DM) with conventional intensive regimens (a very small number), and those patients interested in the technology who were bright and have plenty of money! If the devices were external, I would also consider patients who needed very tight BG control such as pregnant T1’s.
13. As Brown students, we love to think out of the box, so, we are wondering, what is next, Doctor Dempsey? Delve into your wildest dreams and please inform us of what you think scientists will come up with next in order to continue the defeat of Type 1 Diabetes. World-wide screening of non-diabetics for T1 diabetic markers such as GAD-65 and islet cell antibodies. If positive, treat the people at risk with monoclonal antibodies to prevent the development of T1 DM. Rapid advances in continuous BG monitoring to allow accurate testing of BG without forcing patients to constantly check their BG. The ability to grow human beta cells, along with the ability to prevent their destruction once implanted into people with T1 DM.
This interview gave us a very reasoned, practical perspective into the new technology of the artificial pancreas. Although it did confirm our belief (to a certain extent) that some of the articles we read may have been a bit on the optimistic side, again this is only one professional opinion and others may vary. We are still optimistic about this technology.
Next we wanted to interview someone on the opposite end of the spectrum, someone who might be a potential candidate for having an artificial pancreas. Thus we interviewed Ziad Kharbush, a 19-year-old sophomore at Brown University who is a wrestler on the varsity wrestling team and who plans to concentrate in biology. Here is what we found out:
1. When were you diagnosed with type 1 Diabetes? I was diagnosed on March 21, 2005, when I was turning 16.
2. How would you describe the adjustment to type 1 Diabetes? I would definitely say that it’s tedious and aggravating
3. What has been the hardest thing to get used to? The worst part is getting used to the way that it messes with my energy. It’s pretty annoying.
4. What do you think about the artificial pancreas? I think it looks really cool. I remember taking my first shot ever and telling myself, I don't want to have to do this for the rest of my life.
5. Do you plan to use the artificial pancreas? Yes, I’ll use it once it's more efficient and completely safe. I also can’t be playing a full-contact sport (wrestling) if I’m using it.
6. Has diabetes changed your life? If so, how? Yes, I am more careful now. Plus, again, my energy fluctuates so much that everything is a bit harder and more tedious. This is especially difficult since I wrestle, and obviously, you must have a lot of energy when competing in matches, practicing, etc.
7. Since your life has changed, do you think your life will get better with the pancreas? Absolutely yes.
8. What feature are you most excited about with the artificial pancreas? I am most excited about not having to test constantly. It doesn’t seem like a big deal to most people who don’t have diabetes, but it’s always on your mind, and when you have to do it everyday of your life it can get a bit annoying.
Ziad’s optimistic attitude reflects that of many diabetic patients. Soon, hopefully, diabetic patients everywhere will see these hopes fulfilled.
RNAi Summary and Conclusion
14 years ago